
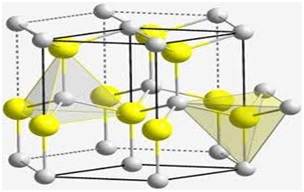
Of all the elements that are available in abundance or scarce, there is a rare element in both the universe and in the earth’s crust. The chemical element is Beryllium. With a symbol ‘Be’ and the atomic number 4, it is one of the lightest of all the light metals and has one of the highest melting points. The element is non–magnetic, has superior thermal conductivity, is resistant to concentrated nitric acid and also resists oxidation in air in normal temperatures. At one point of time, it was known as glucinum meaning ‘sweet’ as many of its compounds are sugary in taste. Beryllium is derived from the Greek word of the mineral beryl, ‘beryllos’. The element is steel–grey, strong, lightweight and a brittle alkaline earth metal.
As mentioned earlier, Beryllium is derived from a mineral beryl. Both emeralds and beryls were known to the early Egyptians but it was realised after the 18th century that they are the same mineral. These two minerals are now called Beryllium and Aluminium Silicate. However, in 1798, M.L Vauquelin recognised the element in beryl and emeralds. Much later in 1828, the metal was isolated by Friederich Wohler and independently by A.B. Bussy by acting Potassium on BeCl2 in a platinum crucible.

Important characteristics of Beryllium include:
- It has one of the highest melting points of 1, 2870 C and a boiling point of about 2, 9700 C.
- The element is steel–grey in colour and is very light.
- It is non–magnetic, has high thermal conductivity and resists attack by concentrated nitric acid.
- The element resists oxidation in air at ordinary temperatures.
- The element is highly permeable to x–radiation.
- It yields neutrons in the ratio of approximately 30 million neutrons per million alpha particles when bombarded.
- Beryllium and its compounds should not be tasted to verify its sweetness as they are toxic in nature.
There are about 30 mineral species that contain Beryllium, out of which Bertrandite, Beryl, Chrysoberyl and Phenacite are the most important. Its precious forms are aquamarine and emerald. The most important commercial sources of Beryllium are beryl and bertrandite. Beryllium was readily available to the industry after 1975 and a majority of the element is now prepared by reducing Beryllium fluoride with magnesium metal.
Primarily, Beryllium is a monoisotopic element as it contains only one stable isotope, 9Be. 10Be, a radioactive cosmogenic isotope is produced in the atmosphere by the cosmic ray spallation of oxygen. The isotope accumulates at the soil surface and thus, its daughter products are used to examine natural soil erosion, soil formation and the development of lateritic soils. Also, it is used as a proxy for measuring the variations in the solar activity and the age of ice cores. Due to the increased solar wind during periods of high solar activity that decreases the flux of galactic cosmic rays reaching the earth, the production of 10Be is inversely proportional to the solar activity. Apparently, 8B has a short half life of about 7x10-17 which contributes to its significant cosmological role. This is because the elements that are heavier than Beryllium could not have been produced by the nuclear fusion in the Big Bang.
Some of the common and important uses of Beryllium are as follows:
- Relatively transparent to X–rays, it is used to make windows for X–ray tubes.
- It is used as a moderator in nuclear reactors.
- In order to make springs, spot–welding electrodes and non–sparking tools, it is alloyed with nickel.
- Its other useful alloys are used in the windshield, brake disks and other structural components of the space shuttle.
- Beryllium oxide, an important compound of Beryllium is used in the nuclear industry and in ceramics.
- It is an important structural material in spacecraft, high–speed aircraft, missiles and communication satellites.
- It is one of the common elements used in gyroscopes, computer parts and instruments that require lightness, stiffness and dimensional stability.
- Beryllium mirrors are used in meteorological satellites which require low weight and long–term dimensional stability.
- Since its mines have magnetic fuzes, its tools are used by naval or military explosive ordnance disposal teams for work on or near naval mines.

A normal human body contains approximately 35 micrograms of Beryllium. However, this amount of the element is not harmful. Chemically similar to magnesium, it displaces itself from the enzymes, which leads to malfunction. Hence, proper care must be taken. Its precautionary measures include:
- No attempt should be made to ingest this highly toxic element.
- Special care must be taken while carrying out any activity in the release of Beryllium dust as prolonged exposure to it may lead to lung cancer.
G Kowledge of | 0 Comments >>
0 Comments
Leave Comment
Your email address will not be published. Required fields are marked.